Describe the Difference Between Endothermic and Exothermic Reactions
Get 1-on-1 help from an expert tutor now. Identify chemical reactions as.

Difference Between Endothermic And Exothermic Reactions
To find out in detail about these reactions one must learn the basic principles behind them.
. Exothermic and endothermic reactions When a chemical reaction occurs energy is transferred to or from the surroundings. In a system energy can do work. A Question 5 5 points Listen REQUIRES WORK.
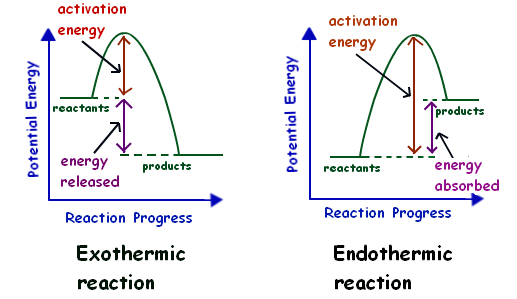
The correct answer would be They are opposites because endothermic reactions require energy and exothermic reactions release energy. 5 rows The main difference between exothermic and endothermic reactions is that an endothermic. The activation energy in an exothermic reaction is energy at the top of the curve minus the energy of the reactants.
Exothermic reactions are reactions or processes that releases energy usually in the form of heat or light to its environment whereas endothermic reactions are reactions that require external energy usually in the form of heat for the reaction to Proceed. 5 rows The major difference between endothermic and exothermic reactions as their names suggest. An endothermic process absorbs heat and cools the surroundings Based on the above definition lets pick a few examples from our daily lives and categorize them as endothermic or exothermic.
On the other hand an exothermic reaction releases energy into the surrounding of the system. An endothermic reaction is that type of reaction which requires or needs energy. A downwards arrow shows that energy is.
End-products in endothermic reaction are less stable. Describe the differences between endothermic and exothermic reactions 2. Describe the difference between an exothermic and endothermic process in terms of a chemical compound.
Activity Summary Exothermic and endothermic are common chemical reactions. In this lab students will measure the changes in temperature during reactions and determine what type of reaction has occurred. Endothermic versus exothermic comparison chart.
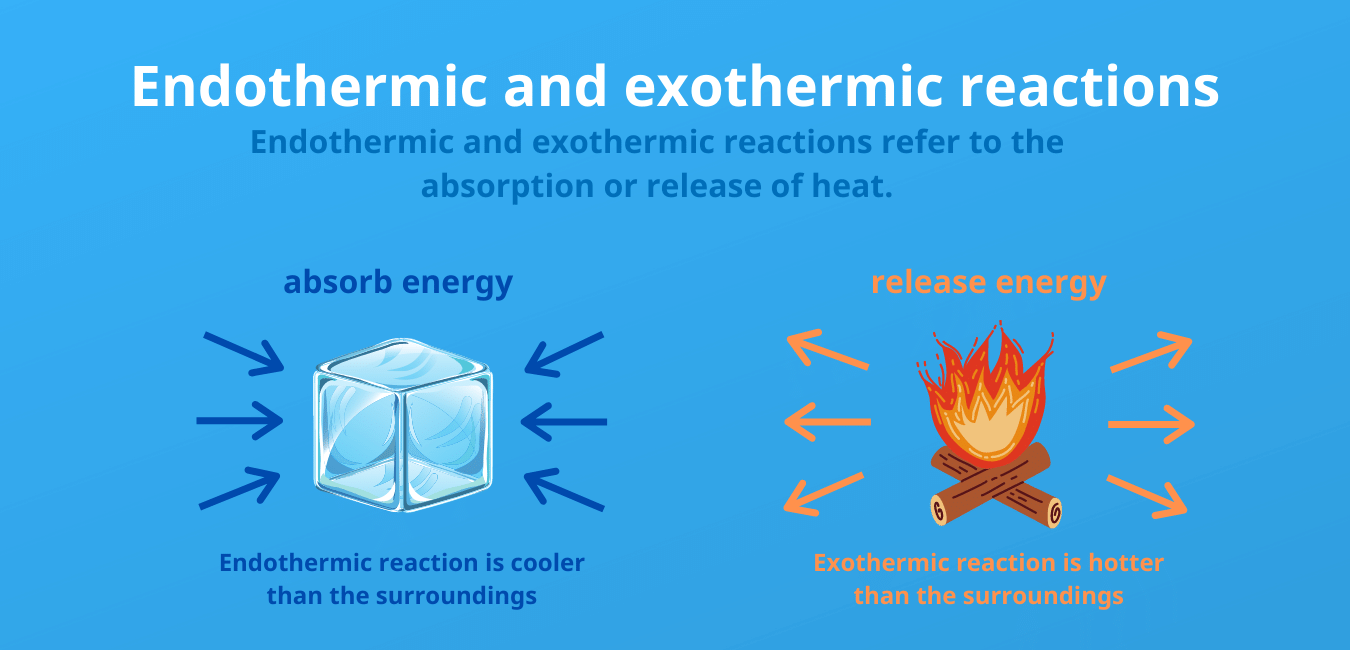
On the other hand an exothermic reaction is the one which releases heat. An exothermic process releases heat causing the temperature of the immediate surroundings to rise. Photosynthesis is a popular example of an endothermic chemical reaction.
Advertisement Answer 38 5 3. An experiment shows that the reaction of nitrogen dioxide with carbon monoxide. Describe the chemical energy difference between exothermic and endothermic reactions.
An endothermic reaction absorbs the energy of its surroundings. Endothermic reactions are those reactions that absorbed energy in the form of heat from the environment whereas exothermic reactions released energy into the surrounding. NO2 g CO g -- NO g CO2g is second order in NO and zero order in CO at 100 C.
They are opposites because endothermic reactions require energy and exothermic reactions release energy. Examples of exothermic reactions are. So energy will have to be provided in case of an endothermic reaction but the exothermic reaction does not require energy.
Grade Levels 9-12 Learning Objectives 1. Burn coal wood fuel plastic among others. Step 4- Set up the table see below and apply the formula for enthalpy change.
This is because energy is given out to the surroundings. It can change into other forms such as heat sound light etc. The main difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding whereas exothermic reactions release energy to the surrounding.
The key difference between endothermic and exothermic reactions is that endothermic reactions absorb energy from the surrounding environment whereas exothermic reactions release energy to the surrounding environment. Step 3- Identify the bond energies of these bonds from Table 73. Step 1- First look at the equation and identify which bonds exist on in the reactants bonds broken.
Chemical reactions are categorized as endothermic and exothermic reactions according to the energy transfer between the system and the surrounding. This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams. Step 5- Since ΔH is negative 23 kcal the reaction is exothermic.
An endothermic reaction has a positive enthalpy difference. An endothermic reaction requires heat. For example when a.
During this process. They are characterized by an increase in enthalpy or positive heat flow that is into the. The endothermic reactions are when the system takes up the energy in the form of light or heat.
Answer in 2-3 sentences. Consider the figure below. The endothermic reactions absorb energy from the surrounding that is in the form of heat.
Describe the differences between endothermic and exothermic reactions. Exothermic reaction The energy level decreases in an exothermic reaction. The digestion of living beings.
There is usually a temperature change. Thus both the reactions are just opposite to each other. Describe the difference between an exothermic and endothermic process in terms of a chemical compound or reaction.
Endothermic reactions are which absorbs energy from outside the system while exothermic reactions give off or releases energy outside the system. The oxidation of metals. Energy is the capacity to do work.
Endothermic reactions are those in which energy is given consumedThe energy can be absorbed in the form of heat light or sound.

7 Difference Between Exothermic And Endothermic Reaction With Examples Viva Differences
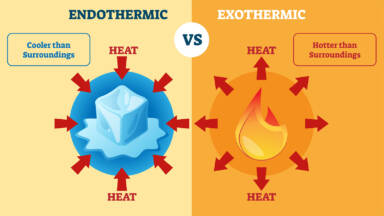
What Does One Mean By Exothermic And Endothermic Reactions Give Examples Cbse Class Notes Online Classnotes123
New Aqa Gcse 2016 Chemistry Endothermic And Exothermic Reactions Teaching Resources
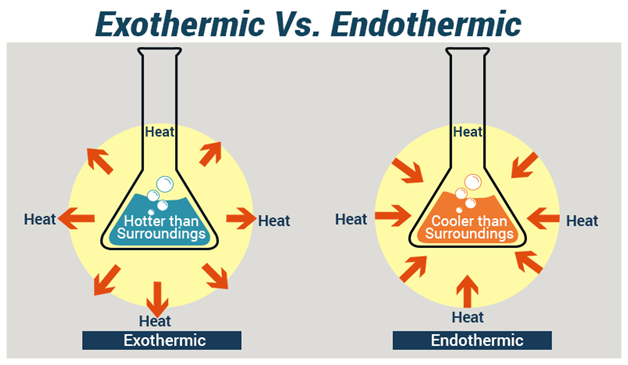
Endothermic Exothermic Reactions What Are They Lessons Blendspace
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Endothermic Reaction Definition Equation Graph Examples

Endothermic And Exothermic Reactions Lab Iteachly Com

Difference Between Endothermic And Exothermic Reactions Compare The Difference Between Similar Terms
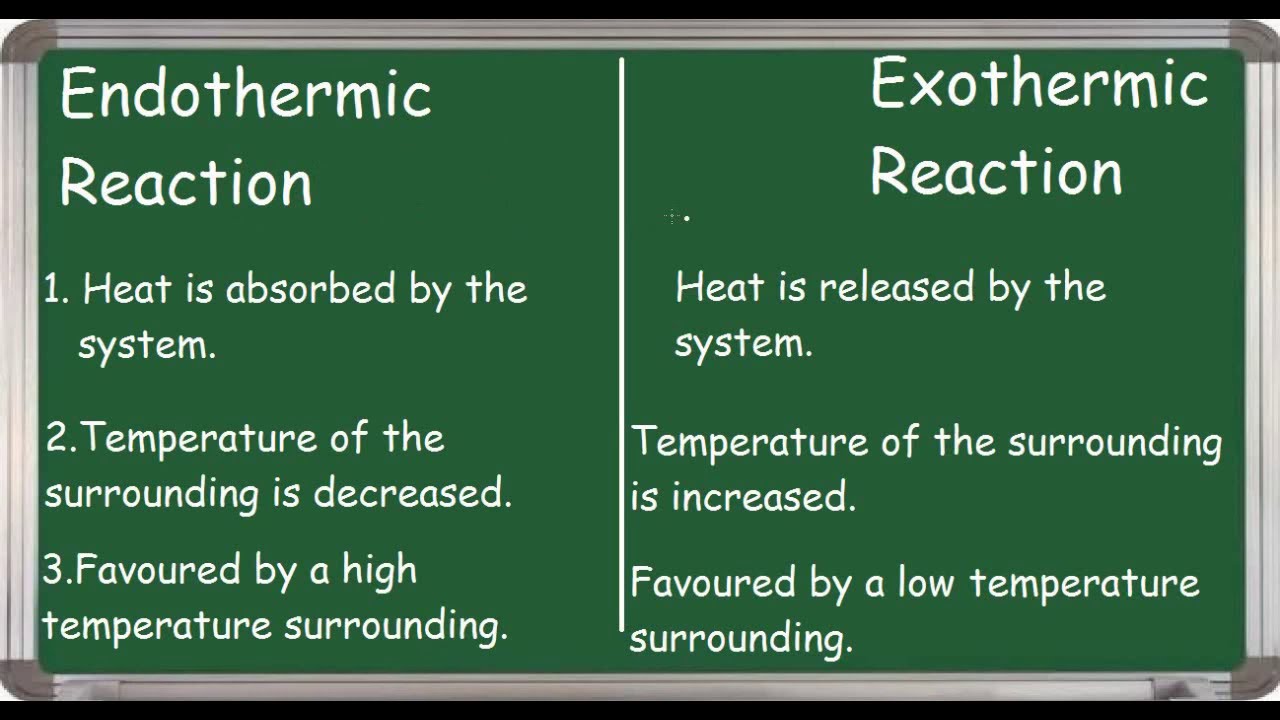
Endothermic Vs Exothermic Reaction Differences Youtube

Endothermic Vs Exothermic Reactions Chemtalk

Exothermic And Endothermic Reactions Labster Theory
Difference Between Endothermic And Exothermic Reactions Definition Properties Examples

Understanding Endothermic And Exothermic Reactions Chemistry Experiments Exothermic Reaction Chemical Reactions
Difference Between Endothermic And Exothermic Reactions With Comparison Chart Bio Differences

Chemistry Alert Endothermic And Exothermic Reactions Facebook
Difference Between Exothermic And Endothermic Reactions Diferr
Determination Of The Enthalpy Instruments And Method

Endothermic Vs Exothermic Reactions Artykul Khan Academy

Exothermic And Endothermic Reactions Definition Examples And Differences
Comments
Post a Comment